» The discovery of bloodless hip reduction was an exquisite solution, in its classical simplicity so very much a product of the simple genius of Adolf Lorenz, a man whose mind was not befuddled with excessive book-learning and theory « (Albert Lorenz writing about his father Adolf Lorenz in the highly readable and amusing biography »Wenn der Vater mit dem Sohne...«).
Definition
▬ Developmental dysplasia of the hip (DDH): Inadequate development of the hip with impaired ossification of the lateral acetabular epiphysis
▬ Congenital dislocation of the hip (CDH): Displacement of the femoral head from its central position in the acetabulum
Historical background
Even Hippocrates (approx. 390 BC) was aware of the existence of a congenital form of hip dislocation [87]. Ambroise Paré (1840) was the first to discover the importance of the role played by the inadequate development of the acetabulum.
Other important milestones in the development of its diagnosis 1846: Wilhelm Roser describes the »ilio-ischeal line«. This line, which passes through the iliac spine, the greater trochanter and the ischial tuberosity, is straight under normal circumstances. In a hip dislocation, however, the trochanter is well above the line, which thus provides a clinical diagnosis.
1895: A new era of diagnosis is opened up with the development of the examination technique discovered by C. Roentgen.
1937: M. Ortolani [63] describes the »Segno d’all scatto« (»clickingsign«).
1962: T.G. Barlow [5] describes a test similar to that of Ortolani.
1980: R. Graf [32] develops the ultrasound hip screening procedure.
Dates relating to treatment
1847: C. G. Pravaz [68]: Longitudinal traction.
1885: A. Lorenz [56]: Immobilization with a hip spica cast in the »frog position«.
1908: K. Ludloff [57]: Open reduction through a medial approach.
1925: H. Hilgenreiner [44]: Introduction of an abduction splint.
1955: W. A. Craig [17]: Introduction of overhead traction.
1957: A. Pavlik [65]: Treatment with a harness.
1968: E. Fettweis [27]: Immobilization with a cast in the squatting position.
Occurrence
Epidemiological figures relating to hip dysplasia should be viewed with caution, since both the screening methods and the interpretation of the findings vary greatly in some cases. Conclusions can be drawn about certain trends, however, on the basis of numerous studies [83].
The dysplasia rate in Central Europe (Germany, Czech Republic, Austria, Switzerland, Northern Italy) used to be from 2–4% until the late seventies. Today it is much lower. The dislocation rate (in historical studies) was 0.5–1%.
In the UK, the USA and Scandinavia, the dysplasia rate is 0.5–1%, and the dislocation rate less than 0.05%.
In a recent study in the UK, 88 dislocations were found in 34’723 neonates (=0,25%) [64]. In Bulgaria, 124 cases of dislocation were found in a total of 20,000 neonates (0.6%) [18]. Dislocation of the hip is practically unknown in black populations. A study investigating almost 17,000 African neonates found not a single case of hip dislocation [24]. The absence of hip dysplasia among the primitive tribes of Africa is thought to be due to the fact that the infants are carried by the mother at the side, resting on the pelvis, or on the back with spread legs.
Other – more northerly located – primitive peoples, for example the Lapps [31] or certain North American Indian tribes [16], tend to wrap their infants tightly and accordingly experience high dislocation rates. Frequencies as high as 5% have been reported. The female:male ratio is approx. 4:1. Regardless of the improved screening methods, a general decline in the incidence is nevertheless apparent.
As with other orthopaedic disorders with a genetic etiological component (for example clubfoot or idiopathic scoliosis), this is probably connected with the increased genetic intermixing of the population. The incidence in alpine countries and Central Europe is approaching that of the English-speaking countries. As we noted in an investigation of pediatric orthopaedic institutions in Switzerland, the decline in the incidence peaked between 1960 and 1980, and the subsequent reduction has been rather less pronounced.
Etiology and pathogenesis
Since the introduction of the ultrasound screening method by Graf [32], we know that, in addition to dysplastic and dislocated hips, there are a large number of immature hips. Percentages as high as 30% have been reported. As part of the evolutionary development of humans, the upright gait led to a widening of the iliac wing to provide additional support for the abdominal organs.
As intelligence developed, the brain and cranium grew in size while, at the same time, the birth canal became narrower. Humans solved this dilemma by bringing their children into the world in a physiologically immature condition. To this immaturity can be added a number of other factors:
▬ genetic,
▬ hormonal and
▬ mechanical.
Dunn [22] differentiated two types of hip dysplasia. The first group shows general joint hypermobility , which manifests itself at birth as hip instability. Girls are predominantly affected (the ratio of boys to girls in this group is 1:12). Hormonal, genetic and constitutional factors play a major role in this group.
The second group is characterized by dysplasia of the acetabulum , without any significant ligament laxity.
Dysplasia is increasingly observed particularly in association with oligohydramnios. This acetabular immaturity is also observed in cases of breech presentation and in connection with other deformities or malformations, e.g. clubfoot, flat feet, facial asymmetries and muscular torticollis. The ratio of boys to girls in this group is only around 1:2, and the left side is twice as likely to be affected as the right side. Mechanical factors associated with the lack of space for the neonate in the uterus play a major role in this group. The consequence is delayed ossification of the lateral acetabular epiphysis, i.e. dysplasia, which leads to secondary dislocation as a result of the inadequate contouring of the acetabular roof. However, the dislocation itself very rarely occurs at birth, but tends to occur secondarily during the course of the first few months of life as a result of the increasing extension in the hip.
As the femoral head starts to be displaced from its central position, this exerts pressure on the lateral acetabular epiphysis, causing ossification and growth to be delayed. Spontaneous normalization is no longer possible by this stage. As the displacement progresses, the femoral head comes out of the acetabulum, usually in a craniodorsal direction. The acetabulum is secondarily filled with fatty and connective tissue. If the femoral head has left the acetabulum, shortening of the iliopsoas muscle will occur. The tendon, which is located right next to and partially fused with, the hip capsule, strangles the capsule and becomes an obstacle to reduction. The elevated position of the femoral head causes shortening of the leg. At the same time, the abductors (particularly the gluteus medius and minimus muscles) and the hip extensors (gluteus maximus) are shortened and weakened.
This leads, on the one hand, to a flexion contracture of the hip and, on the other, to the inability to stabilize the pelvis when standing on one leg. The consequence is an abnormal pelvic tilt that is compensated by hyperlordosis of the lumbar spine.
If the ossification deficit is only slight, the displacement of the femoral head does not occur, and the acetabular dysplasia may heal up spontaneously during subsequent growth as the ossification catches up. There remains the risk, however, that the joint abnormality becomes exacerbated during the pubertal growth spurt [85] (⊡ Fig. 3.167).
Diagnosis
Clinical diagnosis in the neonate
History
▬ Family history (hip dysplasia or premature osteoarthritis
of the hip)
▬ Firstborn child
▬ Amniotic fluid deficiency
▬ Breech presentation .
Hip dysplasia is more common if a corresponding family history exists [45, 64, 83]. Amniotic fluid deficiency and breech presentation are also associated with an increased incidence of hip dysplasia [64, 83].
Clinical examination
Inspection
Asymmetry of skin folds : Pronounced asymmetry of the skin folds can be an indication of unilateral dislocation.
However, since skin folds in the infant are almost never completely symmetrical, this examination is not very informative.
Leg length examination : With the hip and knee flexed at right angles, the thigh on the dislocated side is noticeably shorter (⊡ Fig. 3.152).
Palpation
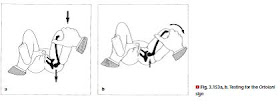
Examination according to Ortolani [63]: The hip and knee are flexed at 90°. Grasp the knee, placing the thumb on the inside of the thigh and the index and middle fingers around the greater trochanter (⊡ Fig. 3.153). First hold the legs in an adducted position and apply gentle pressure in the dorsal direction. Then perform an abduction maneuver, applying slightly greater pressure to the greater trochanter. If the femoral head had been subluxated in the adduction position, a click is perceived as it snaps back into the acetabulum.
Examination according to Barlow [5]: Barlow’s test is
similar to that of Ortolani, but places less emphasis on
the abduction/adduction maneuver, and more on the
thumb pressure. Place the hips in a position of central
abduction. First apply pressure to the greater trochanter
to test the reduction maneuver. Then, from the same
abduction position, try to dislocate the femoral head by
applying pressure dorsally and laterally. If it snaps back
into place, the hip is »dislocatable«. Stabilize the pelvis
with the other hand by placing the thumb on the feet and
encircling the sacrum with the other fingers. The Ortolani
click (⊡ Fig. 3.153) and the Barlow sign remain positive for
approx. 4 weeks in an unstable hip, and cannot be elicited
thereafter regardless of the hip condition.
Examination of abduction
From a position of 90° flexion, the hips are simultaneously
abducted and externally rotated. While the hips of a
healthy neonate can almost always be abducted down to
the examination table, abduction is inhibited in dislocation
or subluxation of the hip in the first 3 months of life.
(⊡ Fig. 3.152).
Examination of the range of motion
Neonates usually show a flexion contracture of around
30–40°. This is a physiological finding, since both hips
are flexed more than 90° within the uterus. Since it is not
possible therefore to examine rotation in the extended
position, rotation is examined in the flexed position in
the usual way.
Ludloff’s dislocation sign : Extension of the knees is not
normally possible if the hip is flexed by more than 90°
because of the tensing of the hamstrings. If the hip is
dislocated however, the knee can be extended in this
position.
For further details on the examination of the hip in
children and adolescents see also chapter 3.3.1.
Radiographic diagnosis
Radiographic diagnosis in infancy is almost completely
irrelevant nowadays since it has been superseded by ultrasound,
an examination that not only involves no radiation
exposure but one that is also more informative. Since the
femoral head center starts to ossify after a year or so, the
diagnosis must then be made radiologically. At this age,
only the AP view is normally recorded ( Chapter 3.2.2).
Other x-ray views do not produce reproducible results
since large sections of the skeleton are still cartilaginous
at this stage and thus not radiopaque. The AP view in
the infant should always be an x-ray of both hips so that the pelvic position and the horizontal situation can be evaluated.
A few guide lines will facilitate a general evaluation of the AP view of an infant (⊡ Fig. 3.154 and 3.155).
▬ The Hilgenreiner line [44] joins the two Y-lines of the triradiate cartilage and thus forms the horizontal on the pelvic view. This horizontal reference line is important because the baby does not always lie completely straight on the x-ray plate.
▬ The Ombrédanne line [62] is drawn from the lateral edge of the acetabular roof, i.e. the lateral acetabular epiphysis (perpendicular to the Hilgenreiner line) and crosses through the Hilgenreiner line to form four quadrants. Normally the center of the femoral head is in the lower inner quadrant. In the early stages of a dislocation, the center is shifted to the lower outer quadrant and, in a high dislocation, to the upper outer quadrant.
▬ Orientation line according to Shenton and Ménard :
Normally the continuation of the medial femoral neck contour forms a smooth arc as it passes through the superior border of the obturator foramen. In a dislocated hip this arc is disrupted because the femoral neck is displaced upwards.
▬ Acetabular roof angle = AC angle or acetabular index [44]: angle between the horizontal (Hilgenreiner line) and the line joining the Triadiate cartilage and the lateral acetabular epiphysis. The average angle at birth is 30°, at 1 year slightly over 20° and at 3 years of age under 20°. ⊡ Fig. 3.156 shows the mean values for this angle in infancy and early childhood, although the accuracy of measurement for this angle is not very great (±5°).
The gap between the femoral head and the radiographic teardrop should not exceed 4 mm up to the age of 4, otherwise instability will be suspected. The radiographic teardrop also deforms over time if dysplasia is present [2].
Arthrography of the hip
Hip arthrography is suitable for evaluating the cartilaginous sections of the hip, the ligament of head of femur and other soft tissues. Although it has become less important since the introduction of ultrasonography, it is still valuable for checking the result of a reduction and the centering of the femoral head after a hip dislocation.
In particular, soft tissue obstructions in the center of the acetabulum are better evaluated by arthrography than by ultrasound.
We use a caudal approach for the arthrography.
The child is placed on a radiolucent table with the legs abducted. From the gluteal fold, a long needle is inserted under sterile conditions and advanced up to the hip under image-intensifier control. 2–3 ml of contrast medium (Jopamiro) are injected. ⊡ Fig. 3.157 shows an arthrogram of the hip.
On the one hand it shows the whole femoral head down to the reflection of the joint capsule and, on the other, the acetabulum from the cranial labrum to the caudal acetabular rim with the transverse ligament. The ligament of the femoral head is also shown. We can readily assess the position of the femoral head in relation to the acetabulum and their demarcation, the shape and position of the labrum and the caudal acetabular rim with the transverse ligament.
It is possible to establish whether intra-articular soft tissue obstructions or an hourglass-shaped constriction of the joint capsule interfere with the deep centering of the femoral head. Additionally, the shortened psoas tendon can leave an impression on the joint capsule and represent an obstacle to reduction. The labrum may not be able to open out correctly or may be pushed in, thereby preventing the deep centering of the head.
Ultrasound examination
At the start of the 1980’s, Graf developed a sonographic screening technique for the infant hip [32] that represented a significant advance in the diagnosis of congenital dysplasia of the hip. Before the era of sonography, the average age for starting treatment for a case of hip dysplasia or dislocation in German-speaking countries was over 8 months [50], compared to the current age of just a few weeks. The main contribution made by Graf was to establish a benchmark for examinations offering a high degree of reproducibility.
Sonography of the hip is performed from a lateral approach, and the ilium as displayed on the image must be parallel with the ultrasound head. If this is not the case, the ultrasound head is positioned either too anteriorly or too posteriorly. A linear scanner is required to produce an image allowing a proper assessment of the situation. The vector scanner frequently used in other investigations is not suitable for hip examination, since it produces a distorted image and the parallel alignment of the iliac margin cannot be evaluated [34]. Suitable frequencies are the 7.5 MHz transducer head for small infants and the 5 MHz head for larger infants.
⊡ Fig. 3.158 presents the findings that can be viewed and interpreted on the ultrasound scan [32].
Graf introduced two angles as a guide to evaluation: alpha angle (angle between the lateral acetabular epiphysis and triadiate cartilage and the lateral margin of the ilium) and beta angle (angle between the lateral border of the ilium and a line joining the lateral acetabular epiphysis and labrum). Graf subsequently proposed a classification taking into account the various conditions of the hip according to the centering of the femoral head, maturation of the bony epiphysis, steepness of the acetabulum and the age of the patient. ⊡ Fig. 3.158 and 3.159 illustrate this classification of the sonographic hip findings, including the morphological criteria, corresponding angles and the need for treatment.
The nomogram in ⊡ Fig. 3.160 allows a classification to be made on the basis of the alpha and beta angles.
Graf ’s ultrasound method has been criticized for a variety of reasons. On the one hand the classification with its combination of figures and letters is not very consistent, since the letters are repeatedly used according to different criteria: types Ia and Ib are differentiated according to the angle, types IIa and IIb according to age and types IIIa and IIIb according to the sonographic density of the cartilaginous epiphysis. The reproducibility of the angle measurements, particularly for the beta angle, is not very great (±10° ) [21]. But probably the most pertinent criticism is that this is a purely static examination with a purely morphological assessment and that an important element of hip dysplasia, i.e. the instability or ligament laxity, is disregarded.
As regards the unreliability of the measurements, both the angular measurements (particularly the beta angle) and the evaluation of the individual morphological criteria (shape of the cartilaginous epiphysis, labrum, etc.) individually show poor reproducibility. If one assesses the overall picture however, the classification is easy, and experienced examiners show substantial agreement when it comes to establishing the type involved. The criticism of poor reproducibility therefore applies only to the consideration of individual parameters in isolation, but not to classifiability and thus the value of the method as a morphological evaluation of the hip.
A more problematic aspect, in our view, is the fact that this is a static rather than a dynamic method. Various authors have proposed other, dynamic, ultrasound examination methods that provide a better assessment of joint instability and ligament laxity. The most popular is that described by Harcke [39] . The problem lies in the lack of standardization of these examinations. The room for subjective evaluation is much greater with these dynamic methods than with the purely morphology-based sonography according to Graf.
When is ultrasound examination appropriate?
There are numerous studies indicating that cases of hip dysplasia are repeatedly overlooked, and require subsequent treatment, with purely clinical screening of neonates [8, 9, 49, 78]. Ultrasound examination therefore seems a useful screening method for all neonates. In Austria this is largely the case in most of the country, while regional variations apply in German and Switzerland.
Several studies also indicate that general screening is more cost effective than treating cases that are discovered too late [9, 51, 85].
The screening of neonates, on the other hand, uncovers a high proportion of immature hips (type IIa) that do not require treatment and usually resolve spontaneously.
A recent Dutch study showed that 95.3% of the type IIa+ and 84.4% of the type IIa- hips develop normally if left untreated [70]. Nevertheless, such hips, accounting for approx. 30% of cases, do need to be monitored [25, 33]. It would be more effective, therefore, to implement general screening at the age of 4 weeks. The problem with this approach is that not all infants can be reliably tracked down at this age, whereas they are already in the maternity ward at birth and have to undergo a comprehensive examination in any case. The ultrasound scan is possible up until the time of ossification of the femoral head center, generally up to the age of 9, or a maximum of 12, months.
If general screening is not available, the ultrasound examination should at least be indicated if certain – broadly interpreted – risk factors are present. The corresponding risk factors are:
▬ a family history of hip dysplasia or coxarthrosis,
▬ premature birth ,
▬ breech presentation,
▬ other skeletal anomalies,
▬ oligohydramnios ,
▬ clinical suspicion of hip dysplasia.
These indications have become generally accepted throughout the German-speaking world, whereas ultrasound scanning is much less widespread in Englishspeaking countries. On the other hand, the incidence of hip dysplasia is also much lower in these countries, where the ultrasound method is only used in a few centers if risk factors are present. In such cases, dynamic examination methods are generally used [8, 58, 85]. Some authors even consider ultrasound scanning to be wholly unnecessary [43].
A certain amount of rethinking is taking place however.
An excellent study from the UK has shown how the treatment costs could be reduced from over £5000 per 1000 neonates after purely clinical screening to £3800 after ultrasound in the presence of risk factors and to £468 with universal ultrasound screening [15]. If the costs of sonographic screening are taken into account, the overall costs are no higher than with purely clinical screening.
There is still some dispute, however, as to whether the ultrasound examination should be performed only if risk factors are present or on a universal basis [46, 64]. There is, of course, no 100% certainty. The above mentioned Dutch study also showed that a very small proportion of initially normal hips became abnormal at 3 months (0.4%) [70].
To sum up: ultrasound examination is a valuable addition to the diagnostic arsenal for investigating the hip in infants. Hip dysplasias can be detected at an early stage with a considerable degree of certainty with the Graf method.
! Universal screening is essential in Central Europe in view of the relatively high incidence of hip dysplasia in these countries.
If screening is not possible, sonographic examination is indicated in the presence of certain, broadly interpreted risk factors. If applied meticulously, the Graf technique provides a highly reliable overall picture, even if the correspondence in respect of individual parameters viewed in isolation is not particularly good.
Treatment
As ultrasound becomes more widespread, concerns are often expressed, particularly by health insurers, about the growing trend of the administration of unnecessary treatments.
! It cannot be stressed too strongly that an immature hip of Graf type IIa does not require treatment. Abduction splinting should not be prescribed simply because of uncertainty about the interpretation of the ultrasound findings since it can also have side effects (femoral head necrosis). Only if a follow-up examination after 6 weeks shows no progress in terms of maturation (type IIa) may such treatment be introduced.
Conservative treatment
The following types of treatment are differentiated:
▬ maturation treatment,
▬ closed reduction,
▬ immobilization.
Maturation treatment
If an immature hip of type IIa or IIc is detected on the ultrasound scan, the femoral head is not dislocated and does not therefore need to be reduced. A maturation treatment with abduction pants or a Tuebingen splint (⊡ Fig. 3.161). The abduction pants were introduced by Frejka in 1941 [28]. These are made of a plastic material and incorporate a rigid bar placed between the legs. The pants hold the legs in abduction and are worn over the infant’s normal clothes. The orthosis cannot be worn continuously since it must be removed for nursing care purposes or when changing the baby’s clothes.
High rates of avascular necrosis were reported during the first few years of abduction splinting [83], at a time when these orthoses were used for reductions. Excessive abductions of up to 90° were also employed. We therefore use the Tuebingen splint developed by A. Bernau [6] for maturation treatment (⊡ Fig. 3.161). This produces less pronounced abduction but greater flexion than standard abduction pants. It is easy to handle and its size can be adjusted to fit the infant. Since it is made from plastic, hygiene is less of a problem than with the Pavlik harness, for example, which is made of fabric.
Reduction methods
We differentiate between the following options:
▬ manual reduction methods,
▬ braces for reduction,
▬ traction methods.
Manual reduction methods
Manual reduction methods are of historical significance only as the associated complication rates were far too high. Manual reductions were described by Lorenz 1895 [56] and Lange in 1898 [53].
Reduction braces
The Pavlik harness [65] incorporates two shoulder straps that cross over at the back and are fastened to a broad chest strap which fastens at the front (⊡ Fig. 3.162). The lower legs are enclosed by stirrup-like straps, with the topmost strap encircling the leg just below the knee.
From the chest strap the shoulder straps continue down to the lower legs. The distance between the chest strap and the lower legs can be adjusted separately by means of buckles at the front and back. The legs are first placed in a flexion position of approx. 110°, which should then be gradually supplemented by increasing abduction. An additional transverse strap can prevent the distraction from exceeding 60°.
This repositioning of the dislocated hip can take a few days in some children, but may require several weeks in others. The dislocated hips reduce themselves spontaneously as a result of the baby’s thrashing about, and no actual reduction maneuver is needed. Naturally, this assumes that the infant possesses normal motor skills.
The use of this harness beyond the age of 9 months is not recommended [83]. In the hands of skilled practitioners, reduction with the Pavlik harness is a reliable method with few complications [11, 40]. Certain authors, how-ever, report a high number of unsuccessful reductions and complications [55, 60, 91].
On the one hand, these findings were very probably the result of inadequate compliance on the part of the mothers. The Pavlik harness is relatively complicated and the numerous straps can be confusing for the parents. For hygienic reasons, the harness has to be changed frequently, and the constant readjustments can be problematic. The main problem is that the harness very easily becomes soiled by the child and cannot then simply be wiped down like a plastic splint. Accordingly, one study has shown that plastic splints are much easier to manage [3].
On the other hand, the Pavlik harness is more suitable for reducing subluxated (Graf type III) hips than completely dislocated (Graf type IV) hips [60]. Another study has also reported a relatively high necrosis rate of 33% after reduction with the Pavlik harness [80].
Traction methods
We make a basic distinction between two methods:
▬ longitudinal traction ,
▬ overhead traction .
Longitudinal traction: Longitudinal traction for reducing the hip is the first known therapeutic procedure and was described by Pravaz in 1847 [68]. It is still used today, in some cases as a home-based treatment. The traction is achieved with plaster strapping affixed to the legs. A board placed beneath the feet is designed to avoid pressure on the malleoli. The traction weight is initially 1/7 of the infant’s weight, but can subsequently be increased to 1/4 or more. The skin should be monitored carefully.
Triangular pants can be used to provide counterforce, or else the foot of the bed can be elevated so that the weight of the body is shifted towards the head. The legs are abducted by approx. 20°. Overhead traction: Overhead traction was introduced in 1955 by Craig [17], and remains a widely used method even today. This traction can also be employed for older children for whom a Pavlik harness is no longer appropriate.
This treatment remains the standard method in our hospital. Overhead traction requires the fitting of two bars at the side of the bed which are linked together above the bed by a crossbar. A weight of 1–1.5 kg is attached to the child’s legs with strapping and exerts traction via a cord that runs over pulleys. The degree of traction should initially be adjusted to produce a flexion of over 90°. The pulleys are then shifted laterally to gradually increase abduction (⊡ Fig. 3.163).

We shift the pulleys so as to achieve an abduction of around 70° after 8–l0 days. By this time spontaneous reduction has occurred in most cases, and this can be checked by arthrography. If the traction were increased to 90° abduction, there would be an increased risk of femoral head necrosis. Reduction with overhead traction must be followed by immobilization, for which we use the Fettweis spica cast (⊡ Fig. 3.164). Traction improves the chances of a successful closed reduction and reduces the risk of avascular necrosis of the femoral head[94].
Immobilization
The following can be used for immobilization:
▬ plaster casts,
▬ splints,
▬ braces,
▬ abduction pants .
Plaster casts
Hip spica in the Lorenz position : This oldest known immobilization treatment described by Lorenz in 1895 [56] fixed the hips in an abduction position of 90° (also known as the »frog position«). We know from large-scale statistical analyses [81] that very many cases of avascular necrosis of the femoral head have occurred as a complication of immobilization in this position. While it was once assumed that this complication was caused by compression of the medial circumflex femoral artery by the posterior acetabular rim during the right-angled abduction, more recent studies have shown that the intraarticular pressure produced by pronounced abduction and internal rotation is excessive and causes constriction of the intra-epiphyseal vessels in the soft cartilage [92].
This also explains why femoral head necroses are less frequent after reductions if the ossification center of the head is present [73]. Immobilization in the Lorenz position is therefore no longer practiced.
» ...Medical specialists also primarily objected to this method because of the need to keep a child in a plaster cast in such a barbaric position for months on end ... « (Albert Lorenz writing about the bloodless reduction and immobilization method developed by his father Adolf Lorenz). Immobilization in the Lange position : In 1898 Lange [53] proposed immobilization in a position of maximum internal rotation and pronounced abduction. This has likewise become obsolete.
Immobilization in a squatting position according to Fettweis : In 1968 Fettweis [27] proposed a treatment of reduction and immobilization in a hip spica in the squatting position, in which the hips are flexed by up to 110–120°, but limiting the abduction to approx. 50° –60° (⊡ Fig. 3.164). Various statistical analyses have shown that the rate of avascular necrosis is much lower, at around 5%, with the squatting position than with the Lorenz position at approx. 15%. The long-term treatment with the Fettweis cast is also very well tolerated by the children. Age is not a relevant factor for this treatment.
Another major advantage of cast treatment is the optimal compliance, which avoids the risk of the child being moved out of the ideal position for prolonged periods.
After a reduction we accordingly always use the Fettweis cast for at least 8 weeks for immobilization purposes.
The cast must be changed after 4 weeks. The cast can be changed under light sedation and does not usually require general anesthesia. The feet do not need to be included in the cast but can be allowed to move freely. The cast need not necessarily be prepared from white plaster and we often use Softcast instead. A sufficiently wide section is cut out of the cast around the buttocks. Self-adhesive plastic inserts that prevent soiling of the cast are available on the market.
Splint treatment
Various abduction splints are used for immobilization purposes. These are particularly suitable as follow-up treatment after immobilization in a Fettweis hip spica.
The Denis Browne splint, introduced in 1948 [10], used to be very popular since it was very easy to manage.
However, since it suffers from the drawback of having been designed for an abduction position of 90° this splint should no longer be used.
Numerous modifications of the Denis Browne splint , with the aim of producing a better position, have been proposed. A well-known example is the Tuebingen splint (⊡ Fig. 3.161), which we tend to use. After a congenital dislocation of the hip, we follow 3 months of permanent immobilization in the squatting cast with a further 3 months of splint treatment. We consider the abduction pants to be inadequate as a maturation treatment after dislocation. The abduction pants are worn over the clothing, while the splint is worn under the clothing. We do not usually administer a maturation treatment exclusively during the night.
The Pavlik harness (⊡ Fig. 3.162) is also suitable for immobilization purposes, although it is not particularly appropriate for use in infants older than 9 months.
Since the Pavlik harness is not very practical for the mother, we only use it occasionally. Various reports in the literature have described failed reduction or subsequent dislocation in the caudal direction after the use of the Pavlik harness [69]. The treatment is only suitable if the parents are cooperative and intelligent.
The Pavlik harness is very popular in English-speaking countries.
Complications after conservative treatment Avascular necrosis of the Femoral head
The commonest and most serious complication of treatment of congenital dislocation of the hip is avascular necrosis of the femoral head. Although it can also occur in untreated hip dislocation, it is very rare in this context.
In most cases, the necrosis is a consequence of treatment and does not result from the dislocation itself. The necrosis can occur in the epiphyseal plate either laterally, centrally or medially (⊡ Fig. 3.165) [83], but most often laterally (⊡ Fig. 3.166).
This results in shortening of the femoral neck, or »head in neck position«, and overgrowth of the greater trochanter. The same shortening of the femoral neck and overgrowth of the greater trochanter is also seen with central necrosis, whereas medial necrosis results in a coxa vara. But the necrosis can also affect the acetabulum.
According to Salter the following 5 factors are important for the diagnosis of femoral head necrosis:
1. Absence of ossification of the femoral head center for more than 1 year after the reduction.
2. Absence of growth of an existing femoral head center for at least 1 year after the reduction.
3. Widening of the femoral neck during the year following the reduction.
4. Increased bone structure of the femoral head center on the x-ray, possibly with subsequent fragmentation.
5. Presence of a deformity of the femoral head and neck after the end of the recovery phase (coxa magna, coxa plana, coxa vara, short femoral neck).
A classification for the severity of the necrosis, presented in ⊡ Table 3.16, was proposed by Toennis [81].
The necrosis rate depends partly on the type of reduction and partly on the immobilization method. ⊡ Table 3.17 shows this correlation on the basis of statistical data collated by the Hip Dysplasia Study Group of the German Orthopaedics and Traumatology Association.
As regards the type of reduction, the overhead method appears to be associated with the lowest rate of necrosis, while the Hoffmann-Daimler brace caused the most circulatory problems. As regards the immobilization on the other hand, the Fettweis squatting position was by far the most favorable method with just 2% of necroses. Necrosis rates of 16% and 27%, respectively, were recorded for the Lange and Lorenz positions. The Pavlik harness was also associated with a fairly low necrosis rate, at 7%. Naturally, the necrosis rate after surgical treatment cannot be compared with the conservative methods since this involves a different population.
» ...The improvement of the Lorenz reduction method did not simply spring from a single individual, like armed Athene from the head of Zeus, but emerged gradually from the cooperation of many scientists... « (Albert Lorenz)
Secondary deterioration
For a long time, doctors assumed that once a hip had returned to normal after treatment it could no longer deteriorate.
But this assumption now needs to be revised. In recent years we have observed several cases in which a normal hip during childhood has deteriorated into a distinctly dysplastic hip during puberty (⊡ Fig. 3.167).
Evidently, premature closure of the triadiate cartilage can occur during puberty so that the acetabulum no longer adequately matches the growth in size of the femoral head.
! Every treated hip must be monitored radiographically until adulthood. X-rays (AP) should – as a minimum requirement, i.e. if no special features are present – be recorded after the start of walking, at the age of 8–10 years and on completion of growth.
Surgical treatment
The surgical treatment of congenital dislocation of the hip serves the following purposes:
▬ open reduction,
▬ joint-correcting measures.
Open reduction
An open reduction (see below) is needed if the hip cannot be reduced in the closed procedure. In the young infant this almost always applies only in cases of teratological dislocation. The longer a hip is dislocated, the more likely it is that secondary changes aggravating any reduction of the head into the acetabulum and impairing the stability of the joint will develop. The femoral head becomes displaced cranially and the capsule is pulled out. The primary acetabulum does not develop correctly and becomes dysfunctional.
If the femoral head strikes the acetabular rim, the cartilaginous epiphysis becomes deformed, possibly resulting in the formation of a cranially-extending channel. Fatty and connective tissue accumulate in the unused hollow space. As the femoral head is displaced, the iliopsoas muscle is pulled upwards and shortened, potentially constricting the capsule tube. The transverse ligament can also protrude like a crescent and thus hinder reduction.
The open reduction can be performed via a medial [57] anterior, lateral or dorsal approach. We prefer the anterior approach. The incision in this case is cranial to the inguinal ligament, subsequently resulting in a very satisfactory cosmetic result. We approach the hip both medially and laterally to the psoas muscle to produce a very good overview. The following factors must be borne in mind during open reduction:
▬ the ligament of the femoral head usually has to be resected,
▬ the acetabulum must be completely cleared out and freed of soft tissues,
▬ the transverse acetabular ligament must be indented,
▬ aponeurotic lengthening of the psoas muscle is often required,
▬ if the femoral head is in a high position, a shortening osteotomy may also be needed,
▬ the widened joint capsule must be sutured and drawn tight.
! Two points are crucially important for the subsequent recovery:
▬ An abnormally high pressure must not develop in the joint.
▬ The femoral head must be deeply centered.
Studies have shown that the deep centering is by far the most important prognostic factor for the subsequent development of the hip, including in respect of the risk of renewed dislocation [12, 29]. More recent MRI studies, however, indicate that the centering is not usually ideal even after a good operation and only returns to normal after 1 year [23]. Since the incidence of femoral head necrosis increases with age we no longer attempt a closed reduction of a high dislocation in children after the first year of life, but proceed directly to an open reduction. In children aged 2 and over an additional shortening osteotomy is usually required, as it is for a high dislocation in children from 1 year of age.
Open reduction is indicated:
▬ in the first year only if closed reduction proves unsuccessful (particularly with a teratological dislocation; chapter 3.2.7); as an alternative an attempt can be made to cut the psoas tendon and the transverse ligament arthroscopically and then retry closed reduction
.▬ in the second year primarily for a high dislocation, i.e. if the femoral head center is higher than the triradiate cartilage or if the closed reduction proves unsuccessful;
▬ from the third year we no longer attempt closed reduction, but proceed directly to open reduction;
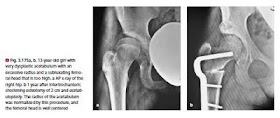
▬ from the fifth year we perform an open reduction only for a unilateral dislocation. The situation should be left as is for a bilateral dislocation (unless a neoacetabulum has formed). The suffering after a reduction attempt is probably greater than if the dislocation is left as is (⊡ Fig. 3.168).
After performing an open reduction we immobilize the hip in a hip spica in the squatting position [27] for at least 3 months. Splinting is then required for at least a further 3 months. The older the child, the longer the follow-up treatment lasts.
Even an experienced orthopaedic surgeon is not always able to reduce the hip in a primarily stable position
with open reduction. The anteromedial capsule, transverse ligament, psoas muscle [14] or a constricted, hourglass- shaped capsule are often responsible for preventing a proper reduction. Sometimes the acetabulum is too small in relation to the femoral head. If the first reduction fails, we generally wait until the child reaches the age of 18 months before making a second attempt. The reduction can then be supported with joint-correcting measures on the acetabulum and femur (see below). Aseptic necrosis occurs as a complication of open reduction in up to 27% of cases [1].
! Every experienced pediatric orthopaedic surgeon has a list of failures that has caused many a sleepless night. Dislocations – and not just teratological ones – can sometimes show anatomical features that prevent the stable centering of the hip, particularly in small children.
Joint-correcting measures (⊡ Table 3.18)
Joint-correcting measures are usually performed secondarily, i.e. not at the same time as the reduction, whether closed or open. Technically correct osteotomies on the pelvis are only feasible from the age of 18 months. Joint correcting measures can be performed essentially at the following sites:
▬ the thigh,
▬ the pelvis.
Femoral osteotomies as joint-correcting measures
Operations on the femur can be performed at the following sites:
▬ intertrochanteric ,
▬ subtrochanteric,
▬ on the greater trochanter (trochanteric transfer).
Intertrochanteric shortening osteotomy : This operation is frequently required for infants with a high dislocation of the femoral head simply in order to move it to a lower position. The femur can be shortened at inter- or subtrochanteric level. The disadvantage of the intertrochanteric osteotomy is the need to chisel the attachment of the psoas muscle off the lesser trochanter. The disadvantage of subtrochanteric shortening, on the other hand, is the substantial tension arising at the shortened psoas tendon, although this can sometimes be offset by aponeurotic lengthening of the tendon. We tend to shorten the femur with the intertrochanteric procedure.
We do not use a step-cut osteotomy for shortening in infants but simply divide the bone smoothly and remove a bone fragment of the desired length. The result is fixed with an infant’s angled plate.
Intertrochanteric varus/derotation osteotomy: Increased anteversion of the femoral neck is frequently seen in connection with hip dysplasia or dislocation. This is only rarely associated with a coxa valga. The valgus position of the femoral neck can often be misinterpreted on the AP x-ray because of the increased anteversion. A correction x-ray with internal rotation can provide information about the precise neck-shaft angle configuration ( Chapter 3.2.8, ⊡ Fig. 3.220).
While an anteverted hip in association with hip dysplasia used to be surgically corrected (at least in Europe) up until the 1970’s, the value of this correction is now disputed.
In the USA, even then, preference tended to be given to acetabular roof reconstruction. In recent years, the belief that acetabular roof reconstruction is better than intertrochanteric osteotomy for improving the biomechan-ics of the joint has also gained acceptance in Europe. The
latter procedure also has the disadvantage that revalgization
frequently recurs during the course of subsequent
growth. At least the intertrochanteric derotation/varus
osteotomy has a secondary effect on the acetabulum, improving
the shape of the acetabulum directly by altering
the pressure distribution [72]. The principle [57] of the
intertrochanteric osteotomy is shown in ⊡ Fig. 3.169. The
result is fixed with an angled plate.
An anteverted hip on its own, without the presence of hip dysplasia, does not constitute an increased risk for osteoarthritis [90]. On the other hand, a retroverted hip is definitely carries a significant risk for early osteoarthritis [84] because of impingement.
Femoral neck lengthening osteotomy : A typical consequence of femoral head necrosis is shortening of the femoral neck with concurrent overgrowth of the greater trochanter, since the trochanteric apophyseal plate is not affected by the necrosis. This configuration will result in abductor weakness of varying severity. A femoral neck lengthening osteotomy can be performed to restore the proper biomechanical configuration [41]. ⊡ Fig. 3.170 shows the principle of this operation, in which
1. the femoral neck length is restored,
2. the lever arm of the abductors is improved by transfer of the greater trochanter and
3. the leg shortening that is usually present is at least partially compensated at the same time.
A lengthening of around 1–1.5 cm can be achieved with this operation (⊡ Fig. 3.171).
The surgeon must be very careful, however, to avoid injury to the vessels that enter the joint capsule and supply the femoral head. Since the pressure in the joint is increased as a result of lengthening of the femoral neck, the procedure is indicated only if the joint conditions are good (largely normal).
Pelvic procedures
As regards the pelvis, the following basic distinction is made between the following types of operation:
▬ Salter osteotomy of the innominate bone,
▬ acetabuloplasty,
▬ Chiari osteotomy of the ilium,
▬ triple osteotomies,
▬ periacetabular osteotomies,
▬ shelf operations.
All of these operations have their own indications and are still commonly performed. Salter’s osteotomy of the innominate bone (ilium) : In Salter’s osteotomy [71], the pelvis is divided above the anterior inferior iliac spine down to the transverse sciatic foramen. The acetabulum is pulled ventrally and laterally. A triangular wedge of bone secures the resulting position. The pivot point for the transfer is the symphysis.
This operation flattens an excessively steep acetabular roof, improves the roof coverage ventrally and
narrows the acetabular angle (see above) (⊡ Fig. 3.172 and 3.173).
The Salter pelvic osteotomy is indicated for an excessively steep acetabulum in a child aged between 2 and 8 years. We hardly ever perform the Salter osteotomy before the age of 2, preferring to wait and see how the situation develops spontaneously. Many mild cases of hip dysplasia improve over time and do not require treatment [26]. Only if the acetabulum is very small, thus preventing a stable closed reduction, do we follow the Salter osteotomy with an open reduction in the same session.
Even in 2-year old patients we frequently await the spontaneous outcome of events despite an acetabular angle of over 30°, since the acetabulum can largely correct itself during this stage of development provided the femoral head is well centered.
Even more important than the acetabular angle for the evaluation is the shape of the lateral acetabular epiphysis and the concavity of the joint surface. If, by the age of 3 years, an acetabular angle of 30°, a flat epiphysis and inadequate concavity of the joint surface are all still present, then the Salter osteotomy is indicated. Since the operation is only feasible while the symphysis remains sufficiently mobile, it is no longer indicated after the age of 8 [38]. A Salter osteotomy can restore the normal hip configuration in small children and even excellent longterm results can be expected. Although one would expect lateralization of the femoral head to occur as a result of the angular movement with the center of rotation in the area of the epiphysis, this does not actually happen in reality [93].
The postoperative management after a Salter osteotomy involves fixation in a hip spica for 6 weeks. The fixation wires are subsequently removed and the child is mobilized. The operation should not be performed on both sides at the same time as a counter support is needed on the opposite side for the rotation of the acetabulum.
The contralateral side should therefore be operated on at the earliest after 4–6 weeks.
While the Salter osteotomy is a relatively simple and tried-and-tested operation, complications can still occur with this procedure. A lesion of the sciatic nerve can occur when the Gigli saw is used in the greater sciatic foramen.
We ourselves have had the misfortune to observe an irreversible partial sciatic nerve lesion (after several operations). Vascular injuries, delayed bone healing and deformation of the iliac crest are other possible complications. Acetabuloplasty : An acetabuloplasty involves a domeshaped osteotomy approx. 1–1.5 cm above the acetabulum in the direction of the triradiate cartilage. The acetabulum is shifted distally by the insertion of a wedge. The main indication for an acetabuloplasty is a non-round or excessively flat acetabulum (⊡ Fig. 3.174 and 3.175).
Weperform an acetabuloplasty most often for neuromuscular hip dislocations . In principle, acetabuloplasty is also a suitable operation for an excessively steep acetabulum in toddlers.
The correction options with acetabuloplasty are better than those with the Salter osteotomy, as the pivot point with the former procedure is nearer the acetabulum (triradiate cartilage compared to the symphysis). However, the risks associated with acetabuloplasty are greater.
Growth disorders in the triradiate cartilage, in particular, can occur. Furthermore, the osteotomy is performed closer to the joint and is technically more demanding than Salter’s innominate osteotomy. We therefore perform the acetabuloplasty primarily in cases of a non-round acetabulum.
Various techniques have been described for the acetabuloplasty, the first originating from Koenig [52]. Spitzy described a technique involving the insertion of a tibial bone graft, which then protruded laterally as in the shelf operation (see relevant section) [76]. The standard technique used nowadays derives from Dega. Pemberton [66] modified this technique and shifted the acetabulum not only distally, but anteriorly as well.
Since we perform an acetabuloplasty primarily for non-round acetabula, the technique is adapted to the initial situation in each case. If the lateral part is too steep, for instance, then this section is turned down accordingly.If the deformation is located ventrally, the correction focuses mainly on the anterior section.
Triple osteotomy : Le Coeur [54] was the first to describe a triple osteotomy of the pelvis. Modifications were subsequently proposed by Hopf [47], Sutherland [79], Steel [78] and Toennis [82]. In all of these osteotomies the ilium, ischium and pubis are divided. The ilium is divided with an osteotome or saw horizontally above the anterior inferior iliac spine, i.e. roughly at the same level as the Salter osteotomy. The ischium and pubic bone are divided differently in the various methods. Le Coeur [54] and Sutherland [79] osteotomied the two bones close to the symphysis. As a result the pivot point was located relatively far from the hip.
Hopf [47], Steel [78] and Toennis [82] proposed osteotomies close to the acetabulum. For children, we employ a modification of the technique described by Steel [78]. Through a separate medial Ludloff approach [59], we cut the ischium much closer to the acetabulum than described by Steel. We osteotomizethe pubic bone by making the cut above the inguinal ligament, while the ilium is divided with the Gigli saw, as in Salter’s osteotomy (⊡ Fig. 3.176 and 3.177).
The triple osteotomy can increase the loading area in the mechanically important anterior and lateral sections of the hip, although this is achieved at the expense of the biomechanically less important caudal medial sections. The biomechanical efficacy of this principle was presented in a recent study [48]. The acetabulum is rotated in a lateral-anterior direction – or if necessary in the individual situation – in a lateral-posterior direction.
Since the acetabulum is then able to swivel over a very wide range, there is also a certain risk of overcorrection.
The triple osteotomy is indicated if the acetabular coverage in the lateral or ventral direction is too small.
This is expressed in a CE angle of less than 10°. The ventral coverage can be checked using the template for spherical hip measurement [42] or on a faux-profil x-ray.
! An important precondition for a triple osteotomy is the need for both the acetabulum and femoral head to be roughly spherical. If this is not the case, the femoral head and acetabulum must be swiveled by the same amount at the same time so that the aspherical congruence is maintained. On the other hand, if the head and acetabulum are spherical but with differing radii, acetabuloplasty is usually the better option.
The triple osteotomy can be performed on children from the age of 8 or on adults. The indications are similar to those for the periacetabular osteotomy, the main difference being that the triple osteotomy can also be implemented with an open triradiate cartilage, which is not the case with the periacetabular osteotomy . We only perform this operation when either definite hiprelated and load-related symptoms are present or if the CE angle is less than 10°. However, the surgeon must carefully establish whether the symptoms are actually associated with poor acetabular coverage rather than an impingement problem.
The latter can also occur after an incorrect reorientation of the acetabulum with a reduction in acetabular anteversion.
The specific technique used is of secondary importance.
The techniques in which the pubis and ischium are divided close to the symphysis are less suitable these days [54, 79], as the pivot point for the swivel movement in such cases is too far from the hip. We use a modified surgical technique according to Steel [78]. Although the Tönnis technique [82] has the advantage of exposing the sciatic nerve via the dorsal approach to the ischium, the resulting scar over the buttocks is not esthetically appealing. Another drawback is the need to turn the patient during the operation.
The most important complication of the triple osteotomy is a sciatic nerve lesion. Fortunately, this is a rare event and the damage is usually transient. The sciatic nerve is at risk during the osteotomies of the ischium and ilium. In over 100 triple and periacetabular osteotomies we have only observed one transient lesion of the sciatic nerve . In theory, the femoral nerve (during the pubic osteotomy) and major vessels are also at risk. A case of premature closure of the triradiate cartilage has also been described [67].
Probably the most common complication is overcorrection or an incorrect orientation of the acetabulum.
Thus, an excessive swiveling maneuver can lead to retroversion of the acetabulum instead of anteversion . Another dangerous situation can occur during lateralization of the acetabulum if the caudal part is not medialized. Incorrect positioning of the acetabulum can change the lever arms of the muscles, potentially resulting in permanent weakness of the abductors in particular.
Another possible complication is necrosis of the acetabulum.
This risk applies particularly if the pubic osteotomy is performed too far laterally, since the vessels supplying the acetabulum from the obturator artery radiate into the acetabulum at the lateral margin of the pubic bone [4]. We have not observed this complication personally.
Another rare event is pseudarthrosis, although no osteotomy is completely free of this risk. Another (rare) complication is the occurrence of periarticular calcifications, which can obstruct movements at a later stage. The complication risks seem to increase with the age of the patient [37].
Periacetabular osteotomy : In the periacetabular osteotomy, the acetabulum is chiseled out without the complete division of all the bones (ilium, pubis, ischium).
This method was first described by Blavier [7]. Wagner [89] modified the procedure to produce a »spherical acetabular osteotomy«, in which the acetabulum is chiseled out spherically approx. 1.5 cm above the cup. On the one hand, this operation is technically demanding while, on the other, the risk of avascular necrosis of the acetabular fragment is very great. Ganz [30] described a periacetabular osteotomy in which the ilium and ischium are not completely divided, but the two cuts are linked by a dorsal osteotomy. This operation can be performed from the ventral side via a single incision. We have accumulated considerable experience with this operation. A precondition is closure of the triradiate cartilage, and the indications are otherwise similar to those for the triple osteotomy.
The advantages of the periacetabular osteotomy over the triple osteotomy:
▬ It can be performed via a single incision.
▬ Sacrospinal ligament not attached to the acetabular fragment, more options for reorientation.
▬ Better stability, since the pelvic ring is preserved intact.
▬ Less fixation required (2 screws), reduced risk of pseudarthrosis
▬ Risk of sciatic nerve lesion slightly less, since the ischium does not need to be divided completely .
A disadvantage is the slightly greater (theoretical) risk of avascular necrosis of the acetabular fragment, although we have not observed this complication in over 300 periacetabular osteotomies. For adult patients, we tend to perform the periacetabular osteotomy according to Ganz. Since the cut crosses the triadiate cartilage this procedure cannot be performed while the child is still growing. In view of the greater general mobility of the pelvis during adulthood, the sacrospinal ligament does not obstruct reorientation of the acetabular fragment as much. A clinical example is shown in ⊡ Fig. 3.178.
The complication risks associated with a periacetabular osteotomy are similar to those of the triple osteotomy. In 30 patients we measured the relevant loading area before and after periacetabular osteotomy using the template described in chapter 3.2. (⊡ Table 3.19).
Preoperatively, the average area was 11.3 cm2, and postoperatively 15.6 cm2, corresponding to an improvement of 38%. Similar results based on computerized measurements have also been reported in the literature [20].
Pelvic osteotomy according to Chiari : This osteotomy
was described by Chiari in 1955 [13]. The technique
involves an oblique osteotomy of the ilium at the level of
the lateral acetabular epiphysis, ascending upwards in the
medial direction, and lateral displacement of the proximal
section of the ilium over the femoral head. The disadvantage
is that the new acetabular roof primarily consists of
bone rather than hyaline cartilage. Moreover, the new acetabular
roof is relatively small in the ventrodorsal plane.
Before the triple and periacetabular osteotomies became popular procedures, the Chiari osteotomy was the only way of improving acetabular coverage in adult hips, particularly in cases where the roof was too short and not too steep (in the latter case acetabuloplasty was also available of course).
We consider that the Chiari osteotomy is almost never indicated nowadays. Even with an aspherical configuration, we prefer the combination of a periacetabular osteotomy with simultaneous intertrochanteric valgization (⊡ Fig. 3.179).
Only for a very small aspherical acetabulum might the Chiari osteotomy still be justified, since it can increase the overall surface area of the acetabulum.
Shelf operation : Augmentation of the acetabulum by the insertion of bone grafts, the so-called »shelf operation«, is a common treatment in English-speaking countries [77]. A similar operation was described by Spitzy as early as 1923 [76]. He wedged tibial grafts in a slot above the lateral acetabular rim. Nowadays, the shelf operation, like the Chiari osteotomy, is only considered as a stopgap measure when the acetabulum is much too small overall. ⊡ Fig. 3.180 shows a combination of the shelf procedure and Chiari osteotomy, in which the graft taken from the femur was used to augment the acetabular roof, wedged against the laterally displaced upper section of the ilium.
Our therapeutic strategy for congenital dislocation of the hip (ultrasound types III or IV according to Graf or radiological dislocation)
Our therapeutic strategy for congenital dislocation of the hip is shown in ⊡ Table 3.20.
References
1. Agus H, Omeroglu H, Ucar H, Bicimoglu A, Turmer Y (2002) Evaluation
of the risk factors of avascular necrosis of the femoral head
in developmental dysplasia of the hip in infants younger than 18
months of age. J Pediatr Orthop B 11: 41–6
2. Albiñana J, Morcuende JA, Weinstein SL (1996) The teardrop in
congenital dislocation of the hip diagnosed late. J Bone Joint Surg
(Am) 78: 1048–55
3. Atar D, Lehmann WB, Tetenbaum Y, Grant AD (1993) Pavlik harness
versus Frejka splint in treatment of developmental dysplasia of
the hip: Bicenter study. J Pediatr Orthop 13: 311–3
4. Bachmann G, Pfeifer T, Spies H, Katthagen BD (1993) 3D-CT und
Angiographie an Ausgusspräparaten von Beckengefäßen: Darstellung
der arteriellen Durchblutung der Hüftgelenkpfanne. Rofo
Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr 158: 214–20
5. Barlow TG (1962) Early diagnosis and treatment of congenital
dislocation of the hip. J Bone Joint Surg (Br) 44: 292–301
6. Bernau A (1990) Die Tübinger Hüftbeugeschiene zur Behandlung
der Hüftdysplasie. Z Orthop 128: 432–5
7. Blavier L, Blavier J (1962) Traitement de la subluxation de la
hanche. Rev Chir Orthop 48: 208–13
8. Boeree NR, Clarke NM (1994) Ultrasound imaging and secondary
screening for congenital dislocation of the hip. J Bone Joint Surg
(Br) 76: 525–33
9. Bon RA, Exner GU (1992) Frühdiagnose der Hüftdysplasie-Argumente
für ein generelles sonographisches Screening in der
Schweiz. Schweiz Rundschau Med Praxis 81: 519–23
10. Brown D (1948) Treatment of congenital dislocation of the hip.
Proc R Soc Med 41: 388
11. Cashman J, Round J, Taylor G, Clarke N (2002) The natural history
of developmental dysplasia of the hip after early supervised treatment
in the Pavlik harness. A prospective, longitudinal follow-up.
J Bone Joint Surg Br 84: 418–25
12. Chen IH, Kuo KN, Lubicky JP (1994) Prognostic factors in acetabular
development following reduction of developmental dysplasia
of the hip. J Pediatr Orthop 14: 3–8
13. Chiari K (1955) Ergebnisse mit der Beckenosteotomie als Pfannendachplastik.
Z Orthop 87: 14
14. Chmielewski J, Albinana J (2002) Failures of open reduction in developmental
dislocation of the hip. J Pediatr Orthop B 11: 284–9
15. Clegg J, Bache C, Raut V (1999) Financial justification for routine
ultrasound screening of the neonatal hip. J Bone Joint Surg Br 81:
852–7
16. Coleman SS (1968) Congenital dysplasia of the hip in Navajo infant,
Clin Orthop 33: 119–28
17. Craig WA, Risser JC, Kramer WG (1955) Review of four hundred
cases of congenital dysplasia and dislocation of the hip. J Bone
Joint Surg (Am) 37: 403–4
18. Darmonov AV (1996) Clinical screening for congenital dislocation
of the hip. J Bone Joint Surg (Am) 78: 383–8
19. Dega W (1964) Schwierigkeiten in der chirurgischen Reposition
der veralteten kongenitalen Subluxation des Hüftgelenkes bei
Kindern. Beitr Orthop Traumatol 11: 642–7
20. de Kleuver M, Kapitein P, Kooijman M, van Limbeek J, Pavlov P,
Veth R (1999) Acetabular coverage of the femoral head after triple
pelvic osteotomy: no relation to outcome in 51 hips followed for
8–15 years. Acta Orthop Scand 70: 583–8
21. Dias JJ, Thomas ICH, Lamont AC, Mody BS, Thompson JR (1993)
The reliability of ultrasonographic assessment of neonatal hips. J
Bone Joint Surg (Br) 75: 479–82
22. Dunn PM (1976) Perinatal observations on the etiology of congenital
dislocation of the hip. Clin Orthop 119: 11–22
23. Duffy C, Taylor F, Coleman L, Graham H, Nattrass G (2002) Magnetic
resonance imaging evaluation of surgical management in
developmental dysplasia of the hip in childhood. J Pediatr Orthop
22: 92–100
24. Edelstein (1964) Congenital dislocation of the hip in the Bantu. J
Bone Joint Surg (Br) 48: 397
25. Eller K, Katthagen BD (1987) Sonographische Verlaufskontrollen
der Hüftdysplasie unter Spreizhosentherapie. Z Orthop 125:
534–41
26. Exner GU, Kern SM (1994) Spontanverlauf milder Hüftdysplasien.
Orthopäde 23: 181–4
27. Fettweis E (1968) Sitz-Hock-Stellungsgips bei Hüftgelenkdysplasien.
Arch Orthop Trauma Surg 63: 38–51
28. Freijka B (1941) Prävention der angeborenen Hüftverrenkung
durch Abduktionspolster. Wien Klin Wochenschr 91: 523
29. Forlin E, Choi H, Guille JT, Bowen JR, Gluttuing J (1992) Prognostic
factors in congenital dislocation of the hip treated with closed
reduction. J Bone Joint Surg (Am) 74: 1140
30. Ganz R, Klaue K, Vinh TS, Mast JW (1988) A new periacetabular
osteotomy for the treatment of hip dysplasias. Clin Orthop 232:
26–36
31. Getz B (1918) The hip in lapps and its bearing on the problem of
congenital dislocation. Acta Orthop Scand Suppl 22: 186
32. Graf R (1984) Fundamentals of sonographic diagnosis of infant hip
dysplasia. J Pediatr Orthop 4: 735–40
33. Graf R, Tschauner C, Steindl M (1987) Ist die IIa-Hüfte behandlungsbedürftig?
Ergebnisse einer Langsschnittuntersuchung
sonographisch kontrollierter Säuglingshüften unter dem 3. Lebensmonat.
Monatsschr Kinderheilkd 135: 832–7
34. Graf R (1992) Hip sonography-how reliable? Sector scanning versus
linear scanning? Dynamic versus static examination. Clin Orthop
281: 18–21
35. Green NE, Lowery ER, Thomas R (1993) Orthopaedic aspects of
prune belly syndrome. J Pediatr Orthop 13: 496–500
36. Guille JT, Forlin E, Kumar J, MacEwen GD (1992) Triple osteotomy
of the innominate bone in treatment of developmental dysplasia
of the hip. J Pediatr Orthop 12: 718–21
37. Hailer NP, Soykaner L, Ackermann H, Rittmeister M (2005) Triple
osteotomy of the pelvis for acetabular dysplasia: age at operation
and the incidence of nonunions and other complications influence
outcome. J Bone Joint Surg Br 87:1622-6
38. Hansson G, Althoff B, Bylund P, Jacobsson B, Löfberg AM, Lönnerholm
T (1990) The Swedish experience with Salter’s innominate
osteotomy in the treatment of congenital subluxation and dislocation
of the hip. J Pediatr Orthop 10: 159–62
39. Harcke HT (1992) Imaging in congenital dislocation and dysplasia
of the hip. Clin Orthop 281: 22–8
40. Harris IE, Dickens R, Menelaus MB (1992) Use of the Pavlik harness
for hip displacements. Clin Orthop 281: 29–33
41. Hefti F, Morscher E (1993) The femoral neck lengthening osteotomy.
Orthopaedics and Traumatol. 2: 144-51
42. Hefti F (1995) Spherical assessment of the hip on standard AP radiographs:
A simple method for the measurement of the contact
area between acetabulum and femoral head and of acetabular
orientation. J Pediatr Orthop 15: 797–805
43. Hernandez RJ, Cornell RG, Hensinger RN (1994) Ultrasound diagnosis
of neonatal congenital dislocation of the hip. A decision
analysis assessment. J Bone Joint Surg (Br) 76: 539–43
44. Hilgenreiner (1925) Zur Frühdiagnose und Frühbehandlung der
angeborenen Hüftgelenkverrenkung. Med Klin 21: 1385–8, 1425–9
45. Hoaglund FT, Healey JH (1990) Osteoarthritis and congenital dysplasia
of the hip in family members of children who have congenital
dysplasia of the hip. J Bone Joint Surg (Am) 72: 1510–8
46. Holen K, Tegnander A, Bredland T, Johansen O, Saether O, Eik-Nes
S, Terjesen T (2002) Universal or selective screening of the neonatal
hip using ultrasound? A prospective, randomised trial of 15,529
newborn infants. J Bone Joint Surg Br 84: 886–90
47. Hopf A (1966) Hüftverlagerung durch doppelte Beckenosteotomie
zur Behandlung der Hüftgelenkdysplasie und Subluxation bei
Jugendlichen und Erwachsenen. Z Orthop 101: 559–86
48. Hsin J, Saluja R, Eilert RE, Wiedel JD (1996) Evaluaton of the biomechanics
of the hip following a triple osteotomy of the innominate
bone. J Bone Joint Surg (Am) 78: 855–62
49. Jones A, Powell N (1990) Ultrasound and neonatal hip screening. A
prospective study of »high risk« babies. J Bone Joint Surg (Br) 72:
457–9
50. Katthagen BD, Mittelmeier H, Becker D (1988) Häufigkeit und
stationärer Behandlungsbeginn kindlicher Hüftgelenkluxationen
in der BR Deutschland. Z Orthop 126: 475–83
51. Klapsch W, Tschauner C, Graf R (1991) Kostendämpfung durch die
generelle sonographische Hüftvorsorgeuntersuchung. Monatsschr
Kinderheilkd 139: 141–3
52. König F (1891) Bildung einer knöchernen Hemmung für den Gelenkkopf
bei der kongenitalen Luxation. Zentralbl Chir 17: 146–7
53. Lange F (1898) Die Behandlung der angeborenen Hüftluxation.
MMW 31: 451, 491
54. LeCoeur P (1965) Ostéotomie isthmique de bascule. In: Chapchal
G (ed) Internationales Symposium über Beckenosteotomie/Pfannendachplastik.
Thieme, Stuttgart
55. Lerman J, Emans J, Millis M, Share J, Zurakowski D, Kasser J (2001)
Early failure of Pavlik harness treatment for developmental hip
dysplasia: clinical and ultrasound predictors. J Pediatr Orthop 21:
348–53
56. Lorenz A (1895) Ueber die mechanische Behandlung der angeborenen
Hüftverrenkung. Zentralbl Chir 22: 153
57. Ludloff (1908) Zur blutigen Einrenkung der angeborenen Hüftluxation.
Z Orthop Chir 22: 272–6
58. Marks DS, Clegg J, al-Chalabi AN (1994) Routine ultrasound
screening for neonatal hip instability. Can it abolish late-presenting
congenital dislocation of the hip? J Bone Joint Surg (Br) 76:
534–8
59. Mayo K, Trumble S, Mast J (1999) Results of periacetabular osteotomy
in patients with previous surgery for hip dysplasia. Clin
Orthop 363: 73–80
60. Mostert A, Tulp N, Castelein R (2000) Results of Pavlik harness
treatment for neonatal hip dislocation as related to Graf’s sonographic
classification. J Pediatr Orthop 20: 306–10
61. Myers S, Eijer H, Ganz R (1999) Anterior femoroacetabular impingement
after periacetabular osteotomy. Clin Orthop 363: 93–9
62. Ombrédanne L (1923) Précis clinique et opératoire de chirurgie
infantile. Masson, Paris
63. Ortolani M (1937) Un segno poco noto e sua importanza per la
diagnosi precoce de prelussazione congenita dell’anca. Pediatria
45: 129
64. Paton RW, Hinduja K, Thomas CD (2005) The significance of at-risk
factors in ultrasound surveillance of developmental dysplasia
of the hip. A ten-year prospective study. J Bone Joint Surg Br
87:1264-6
65. Pavlik A (1957) Die funktionelle Behandlungsmethode mittels Riemenbügel
als Prinzip der konservativen Therapie bei angeborener
Hüftverrenkung der Säuglinge. Z Orthop 89: 341–352
66. Pemberton PA (1965) Pericapsular osteotomy of the ilium for
treatment of congenital subluxation and dislocation of the hip. J
Bone Joint Surg (Am) 47: 65–86
67. Plaster RL, Schoenecker PL, Capelli AM (1991) Premature closure
of the triradiate cartilage: A potential complication of pericapsular
acetabuloplasty. J Pediatr Orthop 11: 676–8
68. Pravaz CG (1847) Traité théorique et pratique des luxations congénitales
du fémur. Baillère, Paris
69. Rombouts JJ, Kaelin A (1992) Inferior (obturator) dislocation of
the hip in neonates. A complication of treatment by the Pavlik
harness. J Bone Joint Surg (Br) 74: 708
70. Roovers EA, Boere-Boonekamp MM, Mostert AK, Castelein RM,
Zielhuis GA, Kerkhoff TH (2005) The natural history of developmental
dysplasia of the hip: sonographic findings in infants of 1-3
months of age. J Pediatr Orthop B 14: 325-30
71. Salter RB (1961) Innominate osteotomy in the treatment of congenital
dislocation and subluxation of the hip in the older child. J
Bone Joint Surg (Br) 43: 518–37
72. Schoenecker PL Anderson DJ, Capelli M (1995) The acetabular
response to proximal femoral varus rotational osteotomy. J Bone
Joint Surg (Am) 77: 990–7
73. Segal L, Boal D, Borthwick L, Clark M, Localio A, Schwentker E
(1999) Avascular necrosis after treatment of DDH: the protective
influence of the ossific nucleus. J Pediatr Orthop 19: 177–84
74. Siebenrock KA, Leunig M, Ganz R (2001) Periacetabular osteotomy:
The Bernese experience. J Bone Jt Surg Br 83: 449–55
75. Siebenrock KA, Schoeniger R, R G (2003) Anterior femoro-acetabular
impingement due to acetabular retroversion. J Bone Joint Surg
Am 85-A: 278–86
76. Spitzy H (1923) Künstliche Pfannendachbildung, Benutzung
von Knochenbolzen zur temporären Fixation. Z Orthop Chir 43:
284–94
77. Staheli LT, Chew DE (1992) Slotted augmentation in childhood
and adolescence. J Pediatr Orthop 12: 569–80
78. Steel HH (1971) Triple osteotomy of the innominate bone. J Bone
Joint Surg (Am) 53: 343–50
79. Sutherland DH, Greenfield R (1977) Double innominate osteotomy.
J Bone Joint Surg (Am) 59: 1082–91
80. Suzuki S, Kashiwagi N, Kasahara Y, Seto Y, Futami T (1996) Avascular
necrosis and the Pavlik harness. J Bone Joint Surg (Br) 78:
631–5
81. Tönnis D (1978) Hüftluxation und Hüftkopfnekrose. Eine Sammelstatistik
des Arbeitskreises Hüftdysplasie. Enke, Stuttgart
(Bücherei des Orthopäden, Bd 21)
82. Toennis D, Behrens K, Tscharani R (1981) A modified technique
of the triple pelvic osteotomy: Early results. J Pediatr Orthop 1:
241–9
83. Toennis D (1984) Die angeborene Hüftdysplasie und Hüftluxation.
Springer, Berlin Heidelberg New York, S 60–3
84. Tönnis D, Heinecke A (1991) Diminished femoral antetorsion syndrome:
A cause of pain and osteoarthritis. J Pediatr Orthop 11:
419–31
85. Tredwell SJ (1992) Neonatal screening for hip joint instability. Its
clinical and economic relevance. Clin Orthop 281: 63–8
86. Tucci JJ, Jay Kumar S, Guille JT, Rubbo ER (1991) Late acetabular
dysplasia following early successful Pavlik harness treatment
of congenital dislocation of the hip. J Pediatr Orthop 11:
502–5
87. Valentin B (1961) Geschichte der Orthopädie. Thieme, Stuttgart
88. van Bergayk AB, Garbuz DS (2002) Quality of life and sports-specific
outcomes after Bernese periacetabular osteotomy. J Bone
Joint Surg Br 84: 339–43
89. Wagner H (1965) Korrektur der Hüftgelenkdysplasie durch die
sphärische Pfannenosteotomie. In: Chapchal G (ed.) Internationales
Symposium über Beckenosteotomie/Pfannendachplastik.
Thieme, Stuttgart
90. Wedge JH, Munkacsi I, Loback D (1989): Anteversion of the femur
and idiopathic osteoarthrosis of the hip. J Bone Joint Surg (Am) 71:
1040–3
91. Wilkinson A, Sherlock D, Murray G (2002) The efficacy of the Pavlik
harness, the Craig splint and the von Rosen splint in the management
of neonatal dysplasia of the hip. A comparative study. J Bone
Joint Surg Br 84: 716–9
92. Wingstrand H (1997) Intracapsular pressure in congenital dislocation
of the hip. J Pediatr Orthop B 6: 245–7
93. Wong-Chung J, Ryan M, O‘Brien T (1990) Movement of the femoral
head after Salter osteotomy for acetabular dysplasia. J Bone Joint
Surg (Br) 72: 563–7
94. Yamada N, Maeda S, Fujii G, Kita A, Funayama K, Kokubun S (2003)
Closed reduction of developmental dislocation of the hip by prolonged
traction. J Bone Joint Surg Br 85: 1173–7